Australia Botulinum Toxin Market Size, Share, Report 2025-2033
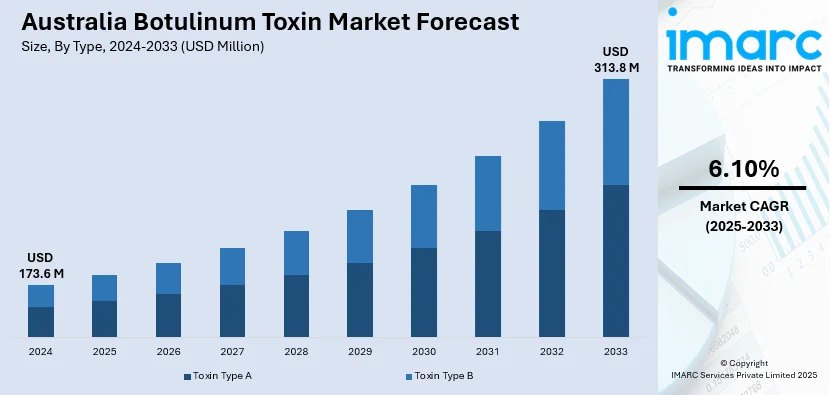
The Australia botulinum toxin market size reached USD 173.6 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 313.8 Million by 2033, exhibiting a growth rate (CAGR) of 6.10% during 2025-2033.
Market Overview
The Australia botulinum toxin market was valued at USD 173.6 Million in 2024 and is projected to reach USD 313.8 Million by 2033. The market is expected to grow steadily during the forecast period of 2025-2033, driven by rising demand for cosmetic procedures, increased aesthetic consciousness, and a growing aging population. Broader therapeutic applications such as treatment for chronic migraine and muscle disorders, along with supportive regulatory approvals and growth in medical tourism, further bolster market expansion.
How AI is Reshaping the Future of Australia botulinum toxin market:
- AI-driven diagnostics and treatment planning enhance precision in botulinum toxin applications, improving patient outcomes and safety.
- Machine learning algorithms optimize supply chain and inventory management for faster and cost-effective distribution of botulinum toxin products in Australia.
- AI-powered regulatory compliance tools facilitate adherence to the Australian Register of Therapeutic Goods (ARTG), reducing unapproved product risks.
- Companies like Evolus, Inc. use AI in market analytics to customize product launches such as Nuceiva (prabotulinumtoxinA), targeting specific consumer groups.
- AI integration with telehealth platforms expands accessibility to botulinum toxin therapies in remote regions, supporting safer administration protocols.
- Real-time AI monitoring supports clinics in maintaining stringent clinical oversight for cosmetic injectables, aligning with regulatory updates by the Medical Board of Australia.
Grab a sample PDF of this report: https://www.imarcgroup.com/australia-botulinum-toxin-market/requestsample
Australia Botulinum Toxin Market Growth Factors
The botulinum toxin market in Australia is said to be driven by a growing industry of cosmetic procedures and increased aesthetics awareness, and the report also noted that a wide spectrum of therapeutic uses such as chronic migraine therapy and the treatment of muscle disorders is expected to drive market growth in Australia. Rising disposable income and an increase in the geriatric population are other major factors contributing towards the market growth. The stringent regulatory approval processes required to expand applications for botulinum toxin along with ensuring product safety among patients also affect the market.
Increased regulation is among the most important drivers of growth in the Australia botulinum toxin market. The new TGA (Therapeutic Goods Administration) regulation directs that no injectable drugs, including botulinum toxin products, can be supplied and administered unless the product is approved and included in the Australian Register of Therapeutic Goods (ARTG). In this way, only registered and safe product is used, with the dangers of unauthorized product importation and off-label prescribing reduced. The increasing regulation is accompanied by a shift in procurement and clinical practice favoring registered ARTG products.
Improvements in clinical governance and regulations of non-surgical aesthetic procedures also encourage market growth. To improve safety, health regulators are stressing credentialing and consent requirements and defining the role of physicians to prescribe and of nurses to administer certain non-surgical treatments. Practice guidelines such as those issued by the Medical Board of Australia are encouraging a new threshold of botulinum toxin practice standards and pushing service providers to comply with certain practice guidelines and to have an increasing level of workforce training and qualification in order to provide higher quality services.
Australia Botulinum Toxin Market Segmentation
Type Insights:
- Toxin Type A
- Toxin Type B
Application Insights:
- Therapeutics
- Aesthetics
End User Insights:
- Hospitals and Clinics
- Dermatology Clinics
- Spas and Cosmetic Centers
Regional Insights:
- Australia Capital Territory & New South Wales
- Victoria & Tasmania
- Queensland
- Northern Territory & Southern Australia
- Western Australia
Key Players
- Evolus, Inc.
Recent Development & News
- July 2025: The Therapeutic Goods Administration (TGA) expanded collaborations with industry stakeholders to strengthen education and regulatory compliance on cosmetic injectables, enhancing patient safety through improved ARTG listing awareness.
- August 2025: Evolus, Inc. announced scaling up operations and launched targeted marketing campaigns for Nuceiva (prabotulinumtoxinA) across major Australian metro areas, improving access to aesthetic botulinum toxin treatments.
- September 2025: Industry data revealed accelerated market growth driven by increased adoption of botulinum toxin products in therapeutic and aesthetic applications, supported by streamlined supply chains and robust regulatory oversight.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
https://www.imarcgroup.com/request?type=report&id=32291&flag=F
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA,
Email: sales@imarcgroup.com,
Tel No: (D) +91 120 433 0800,
United States: +1-201971-6302