Streamlining Clinical Research Through Centralized Data Management Systems
Centralized data management systems streamline clinical research, improving accuracy, efficiency, and compliance across every stage of clinical trials.
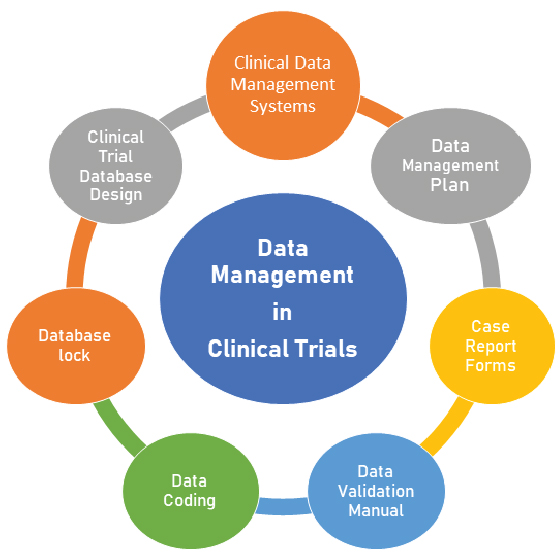
In today’s complex clinical research landscape, the ability to manage vast volumes of clinical trial data efficiently is essential. As studies grow in scale and complexity, data integrity, accessibility, and traceability become critical to success. Centralized data management systems have emerged as the backbone of modern clinical operations, enabling organizations to standardize processes, enhance collaboration, and improve data accuracy.
At Curex Bio, our approach to data management ensures seamless integration across platforms while maintaining the highest standards of compliance and security.
The Growing Need for Centralized Data Management
Clinical trials generate immense volumes of heterogeneous data—from patient information and laboratory results to digital imaging and eCRF (electronic case report form) data. Without a robust data management infrastructure, this information often resides in isolated systems, making analysis and reporting challenging. Centralized systems consolidate this data, allowing researchers and sponsors to gain real-time insights, monitor study progress, and ensure adherence to regulatory requirements.
Effective data management in clinical trials goes beyond storage. It involves structured planning, validation, and quality control to ensure that all collected information meets the standards required for regulatory submission. A centralized data platform provides an integrated environment for collecting, cleaning, and managing data from diverse sources—creating a single source of truth for stakeholders.
Enhancing Clinical Trial Efficiency and Quality
A database management system (DBMS) plays a key role in transforming raw data into actionable intelligence. By enabling data entry, validation, and retrieval within a controlled framework, DBMS technology ensures consistency and traceability throughout the trial lifecycle. Automated processes reduce manual errors, while audit trails maintain transparency for regulatory inspections.
At Curex Bio, our centralized data management system integrates advanced analytics and reporting tools that empower sponsors and CROs to monitor site performance, detect anomalies, and make informed decisions faster. This proactive oversight not only improves data quality but also reduces time to market for new therapies.
Building a Strong Data Governance Framework
A successful centralized system depends on a robust data governance framework. Governance defines the policies, standards, and responsibilities required to manage clinical data throughout its lifecycle. It ensures that information is accurate, complete, and accessible only to authorized users.
Curex Bio emphasizes a governance-first approach, ensuring that every aspect of data management aligns with regulatory guidelines such as ICH-GCP, FDA 21 CFR Part 11, and GDPR. Our framework supports clear accountability, standardized data definitions, and transparent decision-making—critical components for compliance and long-term data reliability.
Integrating Master Data Management for Consistency
In global clinical research, maintaining consistent and standardized information across multiple studies and systems can be a challenge. Master Data Management (MDM) offers a strategic solution by creating a unified and accurate view of critical business data—such as investigator sites, products, and study identifiers.
Curex Bio’s data solutions incorporate MDM principles to synchronize information across diverse platforms. This ensures that teams are always working with the most accurate and up-to-date records. By eliminating duplication and inconsistency, MDM enhances operational efficiency and supports reliable analytics and reporting.
Leveraging Digital Asset Management Systems
As clinical studies become increasingly data-driven, managing digital content—such as protocols, informed consent forms, patient images, and safety documents—requires specialized tools. A Digital Asset Management System (DAMS) provides centralized storage and controlled access for these critical resources.
Curex Bio’s digital asset management capabilities allow secure organization and retrieval of study documents, ensuring version control and compliance. This streamlined approach facilitates collaboration among sponsors, investigators, and data managers, improving communication and reducing administrative burden.
The Role of Technology in Data Integrity and Security
Clinical trial success depends heavily on maintaining the confidentiality and integrity of participant data. Modern data management platforms incorporate advanced encryption, user authentication, and audit features to safeguard sensitive information. With increasing adoption of cloud-based environments, real-time data sharing and monitoring have become more accessible—without compromising security.
Curex Bio implements state-of-the-art technology to ensure that clinical data remains protected at all stages. Our systems are validated according to regulatory standards and undergo continuous monitoring to identify and mitigate potential risks.
Benefits of Centralized Data Management Systems
Adopting a centralized data management model brings multiple advantages:
- Improved data quality: Automated checks and validations reduce errors and inconsistencies.
- Operational efficiency: Centralization minimizes duplication of effort across sites and studies.
- Enhanced collaboration: Teams can access and analyze data in real time, improving decision-making.
- Regulatory compliance: Standardized documentation and traceability ensure readiness for inspections.
- Scalability: The system adapts easily to support multiple trials or therapeutic areas simultaneously.
Future Outlook
The future of clinical research will rely even more heavily on integrated digital ecosystems. Artificial intelligence, machine learning, and automation will further enhance data capture, analysis, and visualization. Organizations that invest in a comprehensive data management infrastructure today will be better positioned to handle tomorrow’s challenges in precision medicine and decentralized trials.