Respiratory Syncytial Virus Vaccines Market: New Targets and Patient Groups
The RSV vaccines market is undergoing rapid transformation, fueled by regulatory approvals, maternal vaccination strategies, and technological innovation.
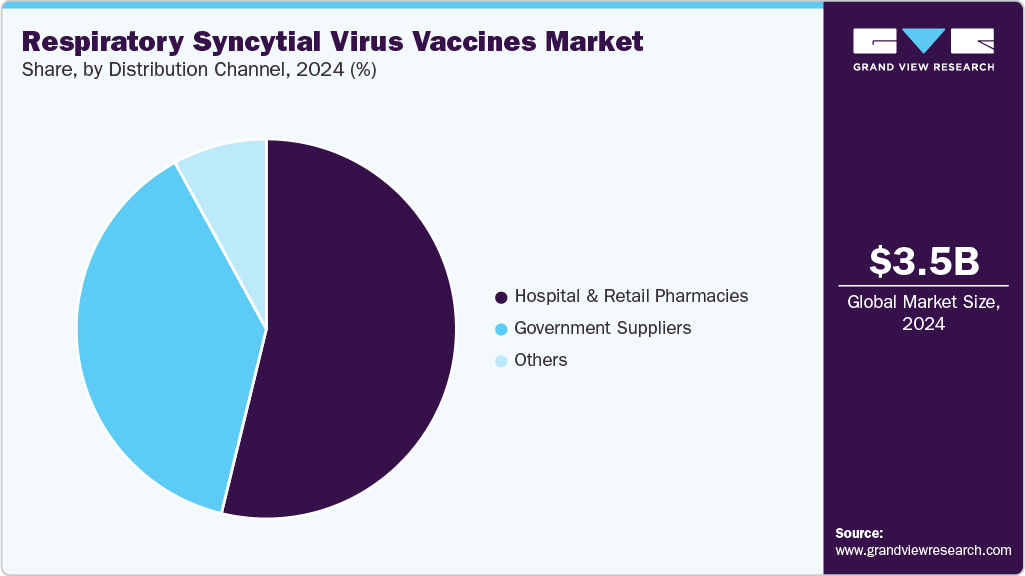
The global respiratory syncytial virus vaccines market size was estimated at USD 3.51 billion in 2024 and is projected to reach USD 28.39 billion by 2030, growing at a CAGR of 42.17% from 2025 to 2030. This rapid expansion is attributed to the rising burden of RSV-related hospitalizations among infants, older adults, and immunocompromised individuals.
As awareness of RSV’s health impact increases, so does the urgency to adopt preventive measures, particularly vaccines and monoclonal antibodies. Advances in mRNA platforms, recombinant proteins, and virus-like particles have significantly expanded the vaccine development pipeline. Historically, management relied mainly on supportive care and monoclonal antibodies for high-risk infants, but the introduction of preventive vaccines has reshaped the landscape.
The market was notably accelerated by the U.S. FDA’s approval of Arexvy (GSK) in May 2023, the first-ever RSV vaccine approved in the United States. Arexvy is indicated for individuals aged 60 and older to prevent RSV-related lower respiratory tract disease. This milestone not only opened the adult vaccine market but also laid the foundation for broader global adoption.
Maternal vaccination is another transformative step, designed to protect infants during their most vulnerable early months. In September 2023, the CDC recommended Abrysvo (Pfizer), a bivalent RSVpreF vaccine, for use in pregnant individuals. Clinical data demonstrated a 57% reduction in RSV-related hospitalizations among infants during their first six months of life, underscoring the vaccine’s impact in reducing pediatric RSV severity.
Key Market Highlights:
- Asia Pacific dominated the RSV vaccines market with the largest revenue share of 30.55% in 2024.
- The U.S. RSV vaccines market is witnessing rapid growth.
- By type, the passive immunization segment accounted for the largest share at 75.02% in 2024.
- By technology, the monoclonal antibodies (mAbs) segment held the largest market revenue share in 2024.
- By targeted population, infants and children led the market with the largest share at 75.02% in 2024.
Download a free sample PDF of the Respiratory Syncytial Virus Vaccines Market Intelligence Study from Grand View Research.
Market Performance:
- 2024 Market Size: USD 3.51 Billion
- 2030 Projected Market Size: USD 28.39 Billion
- CAGR (2025–2030): 42.17%
- Asia Pacific: Largest market in 2024
Prominent Companies & Market Dynamics:
The global RSV vaccines industry is highly competitive, with leading players including AstraZeneca, Sanofi, GSK, Pfizer, Moderna, Icosavax, Meissa Vaccines, Codagenix, Blue Lake Biotechnology, and Merck. Companies are investing heavily in new product development, R&D initiatives, and strategic mergers & acquisitions to strengthen market presence.
For example, in October 2024, Merck reported positive results from its Phase 2b/3 trial (MK-1654-004) evaluating clesrovimab, an investigational monoclonal antibody designed to protect infants during their first RSV season. Interim findings from the ongoing Phase 3 trial (MK-1654-007) were also shared at IDWeek 2024 (October 16–19, Los Angeles), reinforcing the company’s commitment to advancing RSV prevention.
Key Companies:
- AstraZeneca
- Sanofi
- GSK
- Pfizer Inc.
- Moderna
- Icosavax
- Meissa Vaccines
- Codagenix
- Blue Lake Biotechnology
- Merck & Co., Inc.
Explore Horizon Databook, the world’s most comprehensive market intelligence platform by Grand View Research.
Conclusion:
The RSV vaccines market is undergoing rapid transformation, fueled by regulatory approvals, maternal vaccination strategies, and technological innovation. With rising demand from vulnerable populations, strong pipelines, and growing global awareness, the market is positioned for unprecedented growth through 2030.