Clinical Trials Support Software Solutions Market (2025-2033) Europe Market Outlook
The clinical trials support software solutions market is poised for strong growth as the industry increasingly embraces digital platforms to manage complex studies, improve patient engagement, and accelerate drug development.
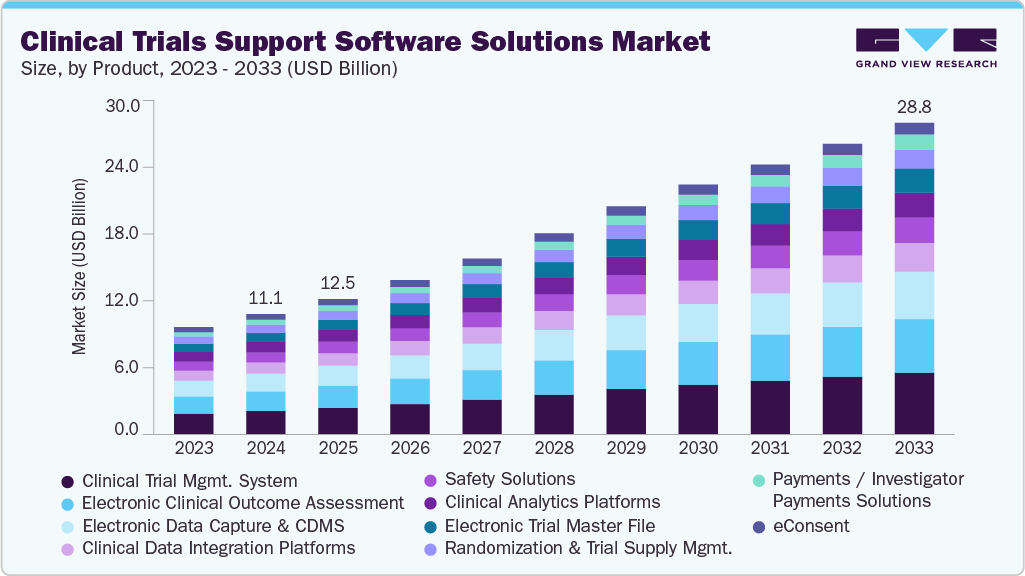
The global clinical trials support software solutions market was valued at USD 11.12 billion in 2024 and is projected to reach USD 28.76 billion by 2033, growing at a CAGR of 10.9% from 2025 to 2033. Market growth is being driven by increasing research and development activities among pharmaceutical and biopharmaceutical companies, which are elevating the demand for digital tools that streamline trial operations and improve efficiency.
The rising need for integrated software platforms is a major catalyst for adoption. These solutions support multiple facets of clinical trials—from study design, data collection, and safety reporting to monitoring and regulatory compliance—helping sponsors optimize timelines and quality outcomes. Additionally, expanding government grants for research, combined with a growing end-use base including CROs, academic institutions, and healthcare providers, is expected to further accelerate market expansion over the forecast period.
Key Market Trends & Insights
- North America dominated the market with a 47.87% share in 2024.
- The U.S. market has grown rapidly, fueled by the rising prevalence of lifestyle-related diseases such as diabetes and cardiovascular disorders.
- By product, the clinical trial management system (CTMS) segment led with 18.68% share in 2024.
- By phase, Phase III was the largest segment in 2024, accounting for 53.38% of market share.
- By end-use, contract research organizations (CROs) held the leading position with 37.24% share in 2024.
Download a free sample PDF of the Clinical Trials Support Software Solutions Market Intelligence Study by Grand View Research.
Market Size & Forecast
- 2024 Market Size: USD 11.12 Billion
- 2033 Projected Market Size: USD 28.76 Billion
- CAGR (2025–2033): 10.9%
- Largest Market (2024): North America
- Fastest-Growing Region: Asia Pacific
Competitive Landscape
Major players in the clinical trials support software solutions market are focusing on expansion, innovation, and strategic partnerships to strengthen their global footprint and enhance product capabilities. Companies are prioritizing usability, interoperability, and AI-enabled analytics to support decentralized clinical trials, accelerate study timelines, and improve data integrity.
Prominent Companies
- Cytel Inc. (acquired by Nordic Capital and Astorg)
- Dassault Systèmes
- Veeva Systems
- IQVIA
- Castor
- Saama
- Oracle
- Parexel International Corporation
- Clario (formerly Bioclinica and ERT)
- RealTime Software Solutions, LLC
- Curebase
Recent Developments
- June 2025: Medpace partnered with Voximetry to integrate its Torch AI-powered dosimetry software into radiopharmaceutical clinical trials, enabling precise, patient-specific dose assessments through a GPU-accelerated, cloud-based platform.
- June 2024: IQVIA launched One Home, a unified clinical trial technology platform designed to simplify and accelerate decentralized clinical trials through integrated tools, services, and centralized data management.
- April 2024: PhaseV and Quanticate partnered to merge AI-driven adaptive clinical trial design with expert biostatistics and data management, helping sponsors improve recruitment, shorten time-to-market, and enhance study quality.
- June 2023: ICON plc released the latest version of its Digital Platform, enabling seamless integration of site, sponsor, and patient services with harmonized data delivery. The platform supports eCOA, direct-to-patient data capture, telehealth visits, eConsent, and digital health technology management.
Explore Horizon Databook – the world’s most comprehensive market intelligence platform by Grand View Research.
Conclusion
The clinical trials support software solutions market is poised for strong growth as the industry increasingly embraces digital platforms to manage complex studies, improve patient engagement, and accelerate drug development. Advances in AI, decentralized trial technologies, and integrated data systems will continue to shape future innovation, enabling more efficient, patient-centric, and regulatory-aligned clinical research worldwide.