Clinical Trial Packaging Market: From Concept to Compliance
The global clinical trial packaging market was valued at USD 3.06 billion in 2024 and is anticipated to grow to USD 5.46 billion by 2030.
The global clinical trial packaging market was valued at USD 3.06 billion in 2024 and is anticipated to grow to USD 5.46 billion by 2030, registering a compound annual growth rate (CAGR) of 10.2% between 2025 and 2030. This expansion is primarily fueled by the increasing number of clinical trials worldwide and the heightened demand for specialized packaging solutions that are temperature-sensitive and comply with regulatory standards.
Stricter regulatory oversight and the rising complexity of clinical trial protocols necessitate secure and adaptable packaging designs. The growth of the clinical trial packaging sector is largely driven by expanding pharmaceutical research and development investments and the surge in novel drug innovations. Regulatory bodies such as the FDA and EMA emphasize rigorous safety and efficacy evaluations, thereby intensifying the need for structured, compliant clinical trials. The surge in personalized medicines, biologics, and vaccines—highlighted during the COVID-19 pandemic—has escalated the volume and intricacy of clinical trials. This trend creates a pressing need for packaging that can support cold chain logistics, small batch sizes, and flexible trial designs.
The market is also benefiting from the increasing complexity of clinical trials, particularly due to the rise of global, multi-site studies and adaptive methodologies. These trials require advanced packaging solutions to maintain drug stability, ensure accurate dosing, and guarantee traceability across various locations and time zones. With a growing emphasis on decentralized and remote trial components, including direct-to-patient drug delivery, packaging solutions must offer tamper-evidence, promote patient compliance, and provide secure labeling. For instance, the integration of smart labels and RFID tags is becoming more widespread, enabling real-time monitoring of conditions such as temperature and humidity throughout the distribution process.
Order a free sample PDF of the Clinical Trial Packaging Market Intelligence Study, published by Grand View Research.
Key Market Trends & Insights
- Regional Dominance: North America led the clinical trial packaging market in 2024, holding over 37.0% of the revenue share. This dominance stems from a strong pharmaceutical R&D infrastructure, rigorous regulatory frameworks, and a high rate of adoption of innovative packaging technologies. The U.S. alone accounts for nearly half of all global clinical trials, driving demand for reliable and compliant packaging solutions.
- Material Preferences: Plastic materials comprised the largest share by material segment in 2024, exceeding 53.0% of revenues. Plastic’s versatility, lightweight properties, and cost efficiency make it the preferred choice for bottles, pouches, and vials. The increasing requirement for sterile, single-use packaging and expanding clinical research in emerging markets further bolster plastic’s leadership.
- Product Segments: Vials and ampoules captured the largest revenue share at over 31.0% in 2024 and are projected to grow at the fastest CAGR of 10.8% through the forecast period. Their versatility in accommodating liquid, lyophilized, and powder drugs makes them a primary packaging format in clinical trials.
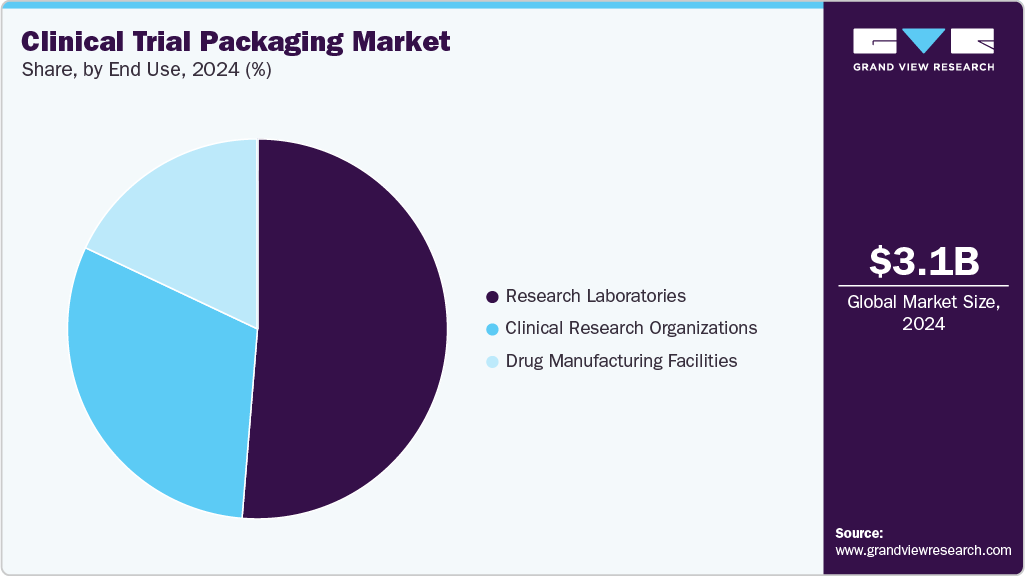
- End Use: Research laboratories accounted for more than 51.0% of the market share in 2024. These laboratories play a crucial role in early-stage drug development, handling preclinical and clinical testing. Their need for specialized packaging to maintain sample integrity and regulatory compliance contributes significantly to the market.
Market Size & Forecast
- 2024 Market Size: USD 3.06 Billion
- 2030 Projected Market Size: USD 5.46 Billion
- CAGR (2025-2030): 10.2%
- North America: Largest market in 2024
- Asia Pacific: Fastest growing region market
Key Companies & Market Share Insights
The clinical trial packaging market features a mix of major global companies and specialized niche providers. These players compete by innovating temperature-controlled and tamper-evident packaging, adhering to strict international regulations (FDA, EMA, ICH), and providing just-in-time and small-batch production tailored for personalized medicine trials. The trend toward outsourcing to contract packaging organizations (CPOs) and the rise of decentralized clinical trials further fuel demand for agile, technology-driven packaging solutions. Companies are heavily investing in smart packaging, sustainability, and automation to maintain competitive advantage.
- In April 2025, Gerresheimer AG introduced silicone-oil- and PFAS-free syringe systems, developed in collaboration with Injecto Group A/S. Available in glass and cyclic olefin polymer (COP), these ready-to-fill syringes reduce particle contamination and medical risks, making them ideal for sensitive biologics and applications such as ophthalmology.
- In October 2024, Nipro unveiled its Direct-to-Fill (D2F) glass vials, utilizing Stevanato Group’s EZ-fill technology. This ready-to-use packaging solution is designed for aseptic fill-finish processes, improving mechanical durability, reducing downtime, and lowering rejection rates during quality control.
Key Players
- Thermo Fisher Scientific Inc.
- Clinigen Limited
- PCI Pharma Services
- Yourway
- WestRock Company
- Oliver
- CCL Industries Inc.
- Sharp Services, LLC
- SCHOTT Pharma
- Gerresheimer AG
- Borosil Scientific
- Nipro
Explore Horizon Databook – The world's most expansive market intelligence platform developed by Grand View Research.
Conclusion
The clinical trial packaging market is poised for robust growth driven by the global rise in clinical trials, increasing complexity of trial designs, and stringent regulatory demands. North America currently leads the market, but Asia Pacific is emerging as the fastest-growing region. Innovations in packaging materials, formats, and smart technologies are essential to address the evolving needs of the pharmaceutical industry, especially in personalized medicine and decentralized trials. As companies focus on safety, compliance, and efficiency, the clinical trial packaging market is set to expand significantly, reaching USD 5.46 billion by 2030 at a CAGR of 10.2%.