Clinical Trial Equipment & Ancillary Solutions Market Service Integration Boost
The global clinical trial equipment & ancillary solutions market is set for steady expansion through 2033, driven by the widespread adoption of decentralized and hybrid trial models, increasing trial complexity, and growing regulatory support for remote research methodologies.
The global clinical trial equipment & ancillary solutions market was valued at USD 3.21 billion in 2024 and is projected to reach USD 6.88 billion by 2033, growing at a CAGR of 8.92% from 2025 to 2033. Market growth is primarily driven by the rising complexity and globalization of clinical trials, which increasingly require specialized, protocol-specific equipment and sophisticated logistical support.
The rapid shift toward decentralized and hybrid clinical trial models has significantly expanded the demand for remote-capable equipment and ancillary services. These modern trial approaches—centered on flexibility, patient convenience, and real-world data collection—rely heavily on wearable sensors, home-use diagnostic kits, remote monitoring devices, and mobile health technologies. As traditional site-dependent trial structures evolve into more patient-centric models, sponsors and CROs must secure validated medical devices for at-home use, temperature-controlled packaging, and logistics systems capable of maintaining sample integrity across geographically dispersed regions.
Regulatory agencies are further accelerating this transition. For instance, the U.S. FDA’s 2023 draft guidance on decentralized clinical trials (DCTs) encourages the adoption of digital health tools, remote assessments, and telemedicine to improve patient access and enhance data quality. These developments have elevated the role of clinical trial equipment providers from simple vendors to integrated service partners responsible for compliance, calibration, real-time tracking, and timely equipment deployment aligned with study protocols.
Key Market Trends & Insights
- North America accounted for the largest market share at 40.46% in 2024.
- The U.S. clinical trial equipment & ancillary solutions industry is expected to experience significant growth over the forecast period.
- By type, the supply and logistics segment dominated the market with a 36.9% revenue share in 2024.
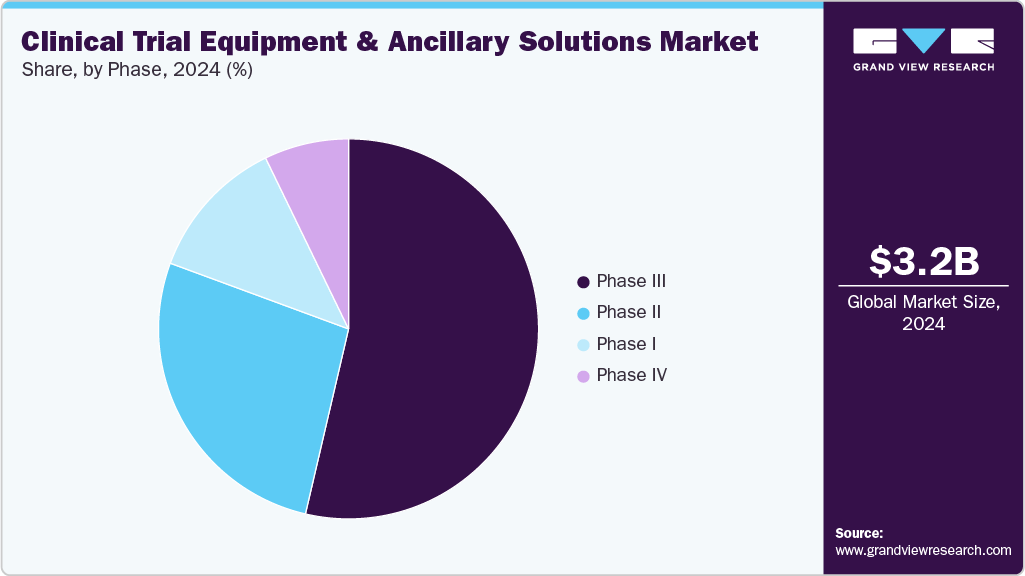
- By phase, the phase III segment captured the largest share in 2024.
Download a free sample PDF of the Clinical Trial Equipment and Ancillary Solutions Market Intelligence Study by Grand View Research.
Market Size & Forecast
- 2024 Market Size: USD 3.21 Billion
- 2033 Projected Market Size: USD 6.88 Billion
- CAGR (2025–2033): 8.92%
- Largest Market: North America
- Fastest-Growing Region: Asia Pacific
Competitive Landscape
Key players are actively pursuing strategic initiatives to strengthen their market presence and expand their service offerings. Common strategies include new service launches, mergers and acquisitions, joint ventures, partnerships, regional expansions, and collaborations. These efforts enable companies to broaden their capabilities, enhance global reach, and maintain a competitive edge amid the rising demand for decentralized trial support.
Prominent Companies
- Ancillare, LP
- Imperial CRS, Inc.
- Woodley Equipment Company Ltd.
- Thermo Fisher Scientific, Inc.
- Parexel International (MA) Corporation
- Emsere (formerly MediCapital Rent)
- Quipment SAS
- IRM
- Marken (UPS – United Parcel Service)
- Myonex
- Yourway
Explore Horizon Databook – the world’s most comprehensive market intelligence platform by Grand View Research.
Conclusion
The global clinical trial equipment & ancillary solutions market is set for steady expansion through 2033, driven by the widespread adoption of decentralized and hybrid trial models, increasing trial complexity, and growing regulatory support for remote research methodologies. As sponsors and CROs require more sophisticated, compliant, and patient-centric equipment solutions, suppliers are evolving into fully integrated service partners. With rising demand for remote monitoring devices, specialized logistics, and validated home-use medical tools, the industry is poised to play an increasingly critical role in shaping the future of clinical research efficiency, accessibility, and innovation.